Hard water is water with a high content of dissolved minerals, namely calcium and magnesium (although iron, aluminum, and manganese can also be found in certain areas). The chemical nature of hard water is due to the content of cations with a +2 charge, especially Ca 2+ and Mg 2+. These ions are not a health problem, but they can react to leave insoluble mineral deposits. This scale can make hard water unusable for many purposes, so there are various means to “soften” water; that is, to remove calcium and magnesium ions from it.
Whereas soft water is purified water in which the only ion is sodium.
Why does water become hard?
Calcium and magnesium dissolved in water are two of the most common minerals that make water “hard. The hardness of water depends on its source. Groundwater that has been in contact with porous rocks containing deposits of minerals like limestone or dolomite will be very hard, while well water is much softer.
At the same time, the hardness varies with the seasons. For example, in winter, especially near the end, there are the highest concentrations of magnesium and calcium in the water. And in spring, during floods, the hardness, on the contrary, decreases.
What affects the hardness of water?
The main difference between hard and soft water is best seen during everyday household chores:
- Hard water causes dirty clothes, dishes with stains and streaks, and bathtubs with lots of plaque and soapy lather.
- Depending on the amount of hardness minerals in the water, the amount of detergent needed to wash or clean changes. Soap becomes less effective because its reaction to magnesium and calcium is not as intense and bubbly.
- Hard water forms scale, which in turn can affect appliances; as a result, appliances start to use more energy.
- Increased hardness can also cause serious damage due to scale buildup inside water pipes, water heater, etc. (when water evaporates as the temperature rises, minerals, precipitate out to form hardened scale).
- Even hair washed in hard water can appear dirty and hard.
- Ions in hard water can also corrode metal pipes due to galvanic corrosion.
- The accumulation of scale and deposits in the pipes results in reduced water flow.
Equipment for water normalization
There are ways to combat elevated mineral content in hard water that will allow households and businesses to enjoy water with better taste and quality. These are reverse osmosis filters and softening units.
In reverse osmosis, the resulting water passes through a thin membrane through which calcium and magnesium cannot pass, they are removed and disposed of. In other words, the water goes from being hard to softer because the ions that cause the water to be hard do not pass through the membrane. You can read how reverse osmosis works here.
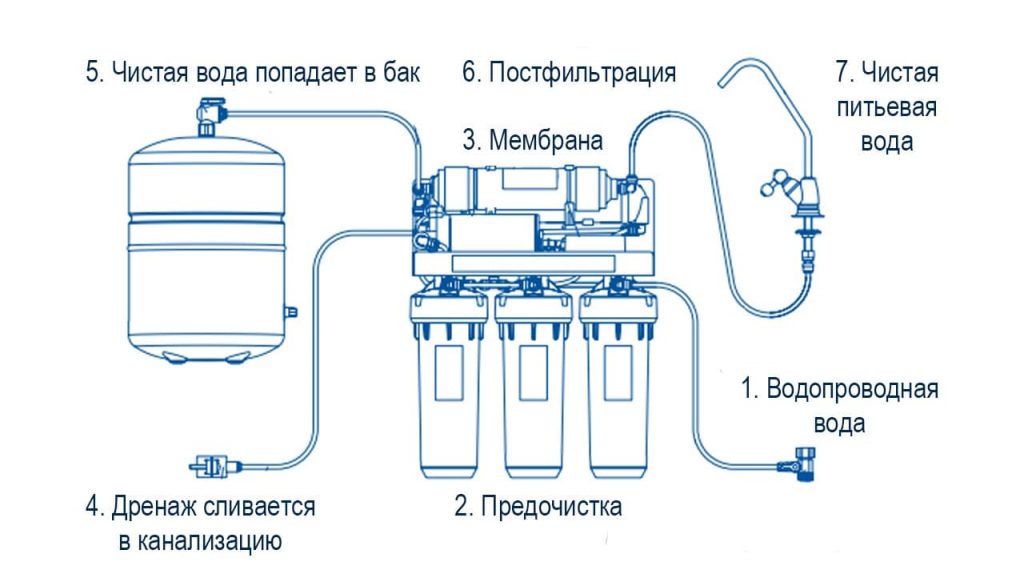
Water softener filters can solve these problems by using ion exchange resins that replace calcium and magnesium ions with sodium and potassium ions. Unfortunately, the disadvantage of this process is an increase in the sodium content of drinking water,
What kind of water do you have?
Many industrial and domestic water users are concerned about water hardness, because when hard water is heated, for example, in a kettle, solid deposits of calcium carbonate, called scale, can form. Whether you use a municipal water system or water from other sources, you can test your water for hardness at home. After all, limescale on household and plumbing fixtures is visible to the naked eye. You may also have felt an unpleasant film enveloping your palms after washing your hands or dishes. You can also have your water analyzed to determine exactly whether the hardness of your water is acceptable and, if necessary, how to filter it.

